Chu Chiheng's research team published a document in Nature Communications to develop a new material for the efficient and complete synthesis of hydrogen peroxide with crystal surface regulated charge separation
2023-03-17
First author: Liu Tian
Corresponding authors: Chu Chiheng, Pan Zhenhua
Communication unit: Zhejiang University
Hydrogen peroxide is an important chemical raw material, which plays an extremely important role in the fields of energy and environment. Among many hydrogen peroxide synthesis technologies, the artificial photosynthesis technology that uses hydrogen and oxygen as the energy conversion carrier and sunlight as the energy injection mode shows great environmental protection advantages and economic value. However, at this stage, organic semiconductors with high attention will inevitably be oxidized and degraded by hydroxyl radicals during the operation of photocatalysis system, so there is a risk of instability during long-term operation.
On February 24, 2022, the team of Chu Chiheng from the School of Environment and Capital of Zhejiang University published a research paper entitled Overall photosynsis of H2O2 by an inorganic semiconductor online in the journal Nature Communication, and developed a green synthesis system of all-inorganic hydrogen peroxide with molybdenum doped single crystal bismuth vanadate as semiconductor and cobalt oxide (CoOx) and palladium (Pd) as cocatalyst. In this work, the quantum yield and energy conversion efficiency of hydrogen peroxide reached 1.2% and 0.29% respectively, breaking the record of inorganic material synthesis of hydrogen peroxide.

First of all, the author prepared CoOx/Mo: BiVO4/Pd with spatially selective supported double promoters using single crystal molybdenum doped bismuth vanadate (Mo: BiVO4) as the semiconductor substrate by means of photodeposition. Taking advantage of the characteristics of the photogenerated charge of single crystal Mo: BiVO4 with selective enrichment of crystal plane, the author selected Co (NO3) 2 and PdCl42 - as the precursors of Co and Pd, and successfully deposited CoOx and Pd selectively on {110} and {010} crystal planes by means of photodeposition (Fig. 1a). According to scanning electron microscope (SEM) and transmission electron microscope (TEM), Co (II) is oxidized by holes and deposited on the {110} crystal plane in the form of CoOx particles, and PdCl42 - is reduced by photoelectron and deposited on the {010} crystal plane in the form of Pd particles (Fig. 1b-f). The selective growth of the two metal species has two purposes on the two crystal planes. One is to provide more active sites for OER and ORR. The other is to strive to significantly change the built-in electric field of {110} crystal plane and {010} crystal plane, and improve the overall separation effect of Mo: BiVO4 photogenerated electrons and holes.
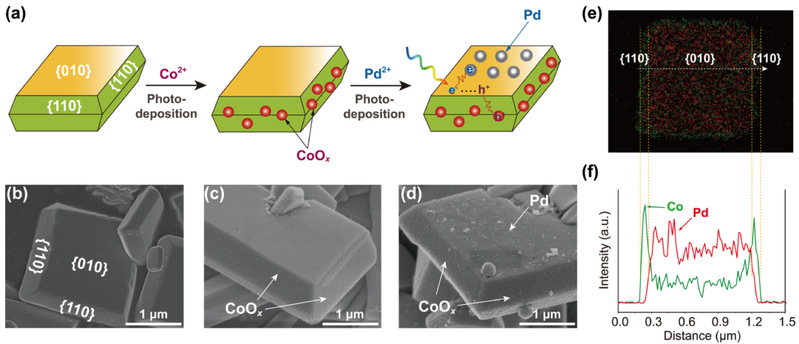
Figure 1. Synthesis principle and microscopic morphology of single crystal bismuth vanadate selectively supported double catalyst
CoOx/Mo: BiVO4/Pd shows excellent performance in the process of photosynthesis of H2O2 compared with the unsupported (Mo: BiVO4), single cocatalyst (CoOx/Mo: BiVO4 and Mo: BiVO4/Pd) and non-selective supported double cocatalyst (Mo: BiVO4-CoOx-Pd). The performance of Mo: BiVO4 was improved by 1.8 times and 53.7 times under the condition of single supported co-catalysts CoOx and Pd; However, the performance of Mo: BiVO4 was improved by 347.6 times when double co-catalysts were loaded at the same time (Fig. 2a). The total solar spectral quantum yield (AQY) of CoOx/Mo: BiVO4/Pd reached 1.2%, and the energy conversion efficiency (STC) reached 0.29%, which is the highest activity of inorganic photocatalysts reported at this stage. The quantum yield at 420 nm is 5.8% (Fig. 2b). The author also noted that the performance improvement times of double promoters are higher than that of single promoters, indicating that the improvement of reaction activity is not a simple improvement of oxidation and reduction reaction activity (Fig. 2c).
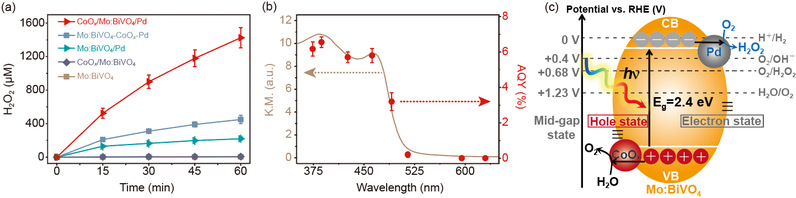
Figure 2. Comparison of hydrogen peroxide production by bismuth vanadate photocatalysis under different loading conditions
After studying the relationship between the reaction activity and the loading condition, the work also studied the relationship between the space charge separation effect, the surface built-in electric field and the loading of different cocatalysts. By comparing the transient absorption spectrum (TAS) attenuation of four different types of materials (Mo: BiVO4, CoOx/Mo: BiVO4/Pd, CoOx/Mo: BiVO4 and Mo: BiVO4/Pd), it can be seen that the carrier life of CoOx/Mo: BiVO4/Pd loaded with double catalysts is much longer than that of other three types of materials (Mo: BiVO4, CoOx/Mo: BiVO4 and Mo: BiVO4/Pd), indicating that the double catalysts not only improve the surface reaction activity, It also synchronously improves the separation of photogenic holes and electrons, and ultimately greatly enhances the energy conversion efficiency of synthetic hydrogen peroxide by artificial photosynthesis.
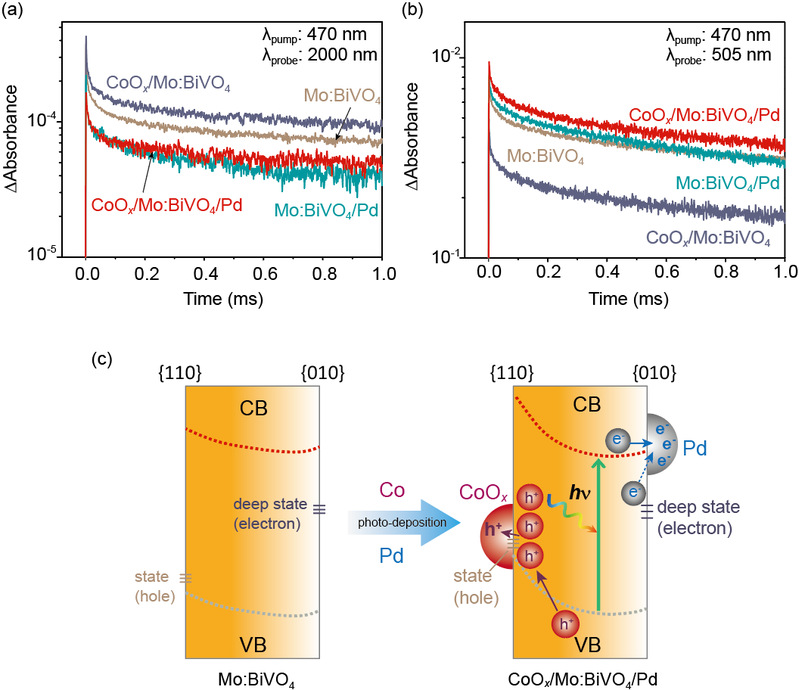
Figure 3. Effect of cocatalyst on space charge decay kinetics and transfer process
By means of controlling the charge transfer mode and surface reaction pathway on the crystal surface, the research has realized the synchronous improvement of charge transfer and surface reaction efficiency in artificial photosynthesis. Under the background of high stability of inorganic photocatalyst materials, the activity of inorganic photocatalyst has been greatly improved, the record of energy conversion efficiency has been refreshed, and a new idea has been provided for the green synthesis of hydrogen peroxide.
Origin link:https://www.nature.com/articles/s41467-022-28686-x
